
CSI HIGHLIGHT OF THE YEAR award:
Structural
CSI Awards celebrate highlights of the year, events such as first-in-mans, trials, studies and approvals, among others, within the field of congenital, structural and valvar heart disease interventions and interventional imaging.
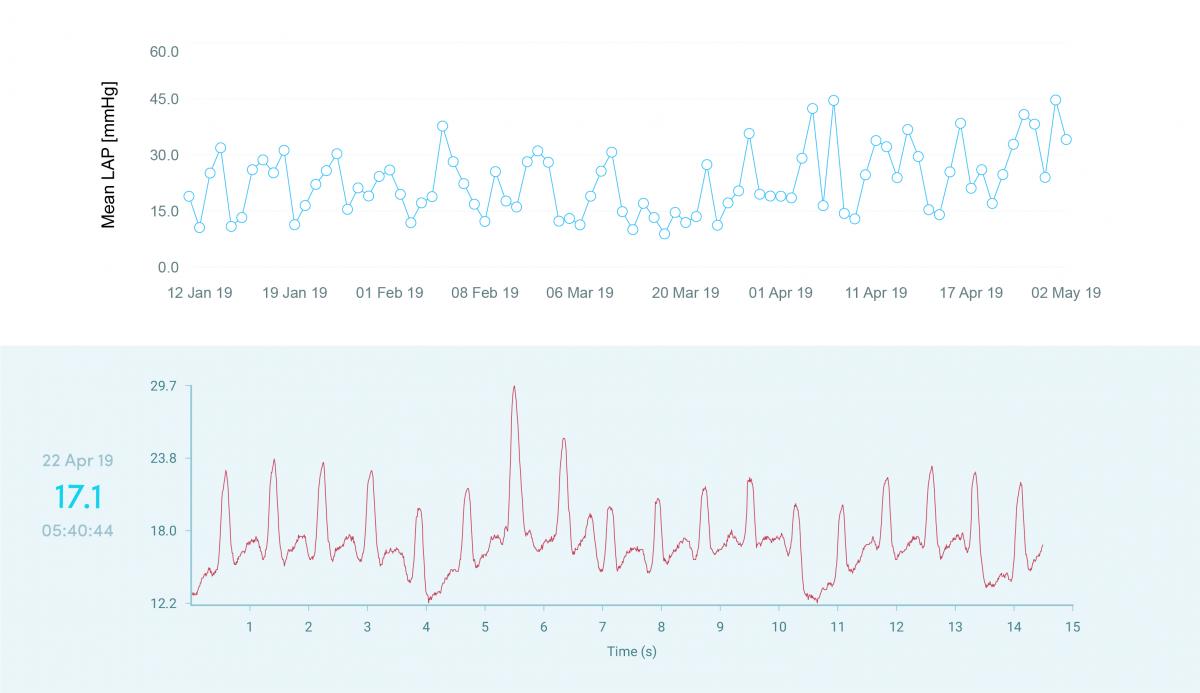
Daily hemodynamic feedback using the world’s first in-heart microcomputer for management of HF patients
vectorious medical | structural
Impact outline: Thus far, two case studies form the first-in-human trial which demonstrates the following: the first patient has great variability in LAP on a daily basis, likely due to rapid changes in preload due to variability in venous capacitance and possibly volume status, accompanied by preload-dependent changes in the severity of functional mitral regurgitation; which was well observed during both TTE and LAP waveform morphology. This patient might be at high-risk for HF hospitalization. As for the second patient: the LAP is well-controlled with little variability.
The V-LAP™ wireless sensor has the potential to play a significant role in the management of HF patients. Clinical studies will demonstrate whether the system enables optimal therapy for HF patients, reduces readmissions and detects co-morbidities in a personalized manner. Daily hemodynamic feedback from the heart’s left atrium opens up a new era in the management of HF patients and scientific understandings.
Background/supporting information: Heart failure (HF) is a leading cause of hospital readmissions. Despite advancements in diagnostic and therapeutic modalities, the rate of HF hospitalizations has not changed substantially throughout recent years. While non-invasive monitoring approaches have generally failed to reduce the risk of HF hospitalizations, intracardiac or pulmonary artery pressure guided management had succeeded. The V-LAP™ system is a left-sided pressure monitoring medical device based on a novel digital, wireless and miniature implantable sensor which measures and transmits high resolution left-atrial pressure (LAP) waveform morphology in the ambulatory setting. The V-LAP™ sensor is able to directly measure absolute pressures in the left atrium with a high-degree of fidelity, compensate for pressure drift, transmit wirelessly daily hemodynamic feedback from the heart, and detect common comorbidities such as atrial fibrillation and mitral regurgitation. The wireless sensor is implanted using a trans-septal approach, under angiographic and echocardiographic guidance. The V-LAP™ system includes an external unit, which powers the implant remotely and collects data via radio frequency communication upon activation, designed to be operated on a daily basis.
* * *
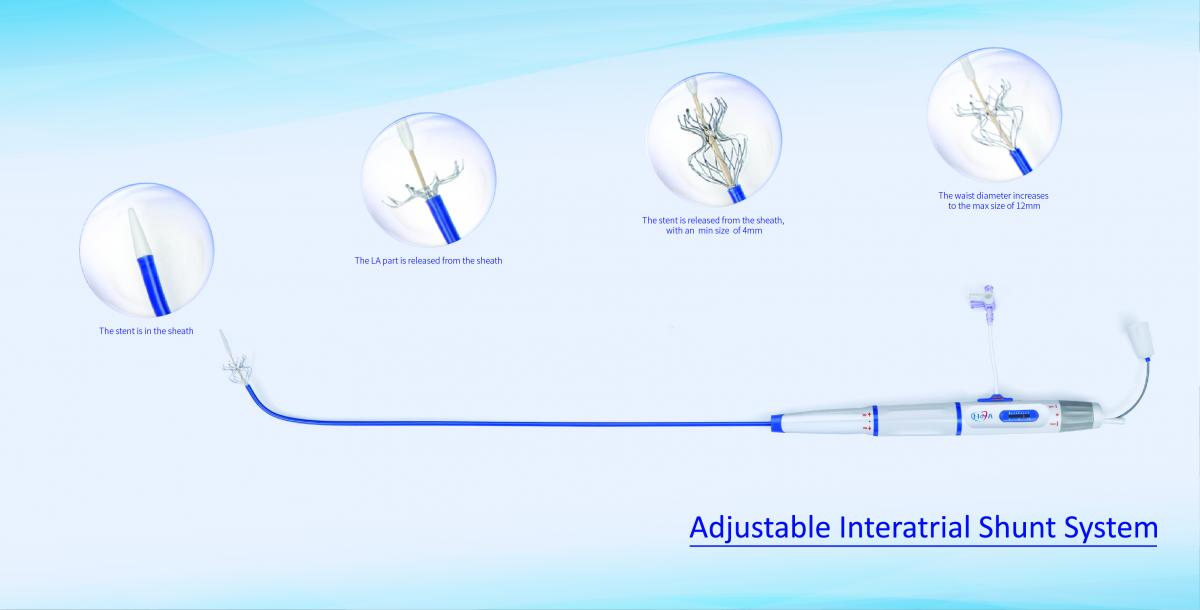
NoYA-new adjustable interatrial shunt system opens a new era of heart failure treatment
venus medtech | Structural
Impact outline: Man-made “atrial septal perforation” is a new concept in the treatment of heart failure. The atrial shunt devices like V-WAVE (Israel) and Isad (USA) have been proved to be safe and effective through small clinical trials. But both devices have fixed shunt apertures and carry the risk of blood clots from permanent implants. NoYa™ makes up for the above deficiencies and marks an innovation in the field of heart failure treatment.
The advantages of NoYA™ lie in the simplicity of the procedure and the avoidance of an implant, which renders anticoagulation or antiplatelet therapy unnecessary. In addition, we need to consider whether the blood shunting aperture can adopt standardized design. Some people think that the shunting aperture must be set according to the pressure difference between the left and right atrium. The coming problem is that the pressure level difference at rest is different from that at motion. Therefore, we do not have a good answer for the aperture size. However, the size of the aperture created by NoYA™ is adjustable, which is one of the advantages of NoYa™ over V-wave and ISAD.
Background/supporting information: The population’s ageing process is increasing the number of people with heart failure worldwide, leading to the growing market demand for heart failure treatments. In clinical practice, when we encounter some patients with dyspnea symptoms, can we make a definite diagnosis of heart failure? Some companies in our country are trying to diagnose early heart failure by measuring fluid retention or rale change in the lungs. Once heart failure is diagnosed, the best way to treat it is to divide stagnant blood from the left atrium to the right atrium, which is how NoYA™ works. That means, in the intervention condition, through the electrode stent support and radiofrequency ablation, we artificially cause "atrial septal defect", so that blood can flow from the left atrium to the right atrium.
* * *
Initial human experience with the OMEGA™ LAA occluder in
patients with atrial fibrillation and contraindication to
long-term oral anticoagulant therapy
Eclipse medical | STRUCTURAL
Impact outline: The OMEGA™ Left Atrial Appendage Occluder (Eclipse Medical, Ireland) is a novel device designed for percutaneous left atrial appendage (LAA) closure to reduce thrombo-embolic events in patients with non-valvular atrial fibrillation and high bleeding risk. The OMEGA™ device consists of a cup for fixation in the LAA and a disk to seal the entrance from the left atrium to the LAA. A connecting waist articulates the cup and the disc, retaining high flexibility. The dual layer, self-expandable cup and disc are made from a nitinol wire mesh coated with platinum. The disc has a polypropylene fabric securely sewn internally to add to the occlusive aspect of the device. Device sizes range from 18 to 30 mm, suitable for use in patients with LAA landing zone diameter from 13 to 26 mm. The OMEGA™ LAA Occluder is indicated for the usage in combination with the 14Fr OMEGA™ Delivery System (Vascular Innovations Co. Ltd., Thailand).
Background/supporting information: Six patients were selected for implantation of the OMEGA™ LAA occluder. The mean age was 78±10 years, 50% were male, mean CHA2DS2-VASc score 3.5% ± 1.6% and HAS-BLED score 2.5% ± 0.7%. Procedures were performed under local anaesthesia and sedation, guided by TEE. The implantations were done by femoral venous approach and transseptal puncture. All patients received the OMEGA™ device successfully with a mean total procedure time of 39±12 minutes and with a mean time of 19±7 min of the OMEGA™ delivery system in the body. All patients received the OMEGA™ device size as determined by pre-procedural planning, and only one device was used in each patient. There was no procedural incidence of hemopericardium, device embolization, vascular complication or thrombo-embolic events. Complete closure of the LAA was achieved in all patients. The First-In-Human implantations of the OMEGA™ LAA occluder demonstrate a high procedural safety and performance profile. A larger study of longer-term safety and efficacy is ongoing.
* * *
Three-dimensional printed models of the LAA
helping to secure safe and successful first-in-human
implantations of the novel OMEGA LAA occluder
ECLIPSE MEDICAL | STRUCTURAL
Impact outline: The OMEGA™ Left Atrial Appendage (LAA) occluder (Eclipse Medical, Ireland) is a novel device designed for percutaneous LAA closure aiming to reduce thromboembolic events in patients with non-valvular atrial fibrillation and a high bleeding risk. The OMEGA™ device consists of a cup for fixation in the LAA and a disk to seal the entrance from the left atrium to the LAA. After pre-clinical testing of the OMEGA™ device, thorough pre-procedural planning may ensure safe and successful First-In-Human (FIH) implantations.
Background/supporting information: Based on bench test implants in 20 different human LAA-models, it could be determined that the ‘landing zone’ of the OMEGATM LAA occluder cup should be measured at a depth of 12 to 15 mm, as measured from the LAA ostium. Moreover, it could be determined that a 15% to 30% compression rate on the cup would be optimal and that a slight traction on the disk into the LAA should be recommended. Following case preparation with patient-specific 3D-printed LAA models, the FIH implants of the OMEGATM LAA occluder (n=5) were all successful without any procedural complication, no need for change to another device size during implantation, and complete sealing of the LAA at control echocardiographic follow-up. In the 3D-models, the mean device compression rate was measured to be 22.0 ± 5.2% as compared to a mean device compression rate of 26.8 ± 5.8% in the actual implants, as measured at fluoroscopy (i.e. absolute difference in compression rate between 3D-models and FIH cases of only 4.8 ± 2.1%). The use of 3D-printed LAA models has been useful in determining well-founded implant instructions for the novel OMEGATM LAA occluder – before the actual FIH cases – and has helped obtaining safe and successful FIH implantations of this novel medical device.
* * *
True3D is the first interactive mixed reality software platform for improved medical imaging that facilitates more precise and personalized surgical planning
Echopixel | STRUCTURAL
Impact outline: Anatomic understanding. Current 2D visualization techniques have limitations representing 3D relationships among organs and tissues. To interpret medical images, a physician must mentally integrate a series of 2D images and extract the relevant 3D relationships among organs and tissues. This involves mapping two or more 2D images on to an orientation free, 3D mental reconstruction of the anatomy to understand the important features of the lesion. The True3D Viewer provides a new visualization technique that facilitates better 3D visualization to define anatomical relationships and details that aid and enhance radiological imaging and surgical planning.
Background/supporting information: The True3D Viewer software uses a computer , a 3D stereoscopic display, head tracking equipment, and a hand-controlled stylus. True3D images created by this system have the following features:
Stereoscopic 3D Parallax: While the viewer wears Stereoscopic 3D polarized glasses, the images from the left and right eyes fuse into a single True3D image that fully conveys depth and perspective as a function of parallax — the relative difference in horizontal positions of an object seen with the left and right eyes.
Motion Parallax: By tracking the motion and position of the user’s head, each True3D image is rendered to match the user’s left and right eye position. Thereby providing correct perspective of the objects within the image.
Prehension: Any object in the True3D image can be manipulated using a stylus that tracks the motion of the operator’s hand. The correct relations among anatomical structures is maintained regardless of the orientation of the image.
True3D image that contain the 3 elements described above creates a real time, interactive, holographic experience allowing the user to visualize, comprehend, and interact with body parts in open 3D space.
* * *
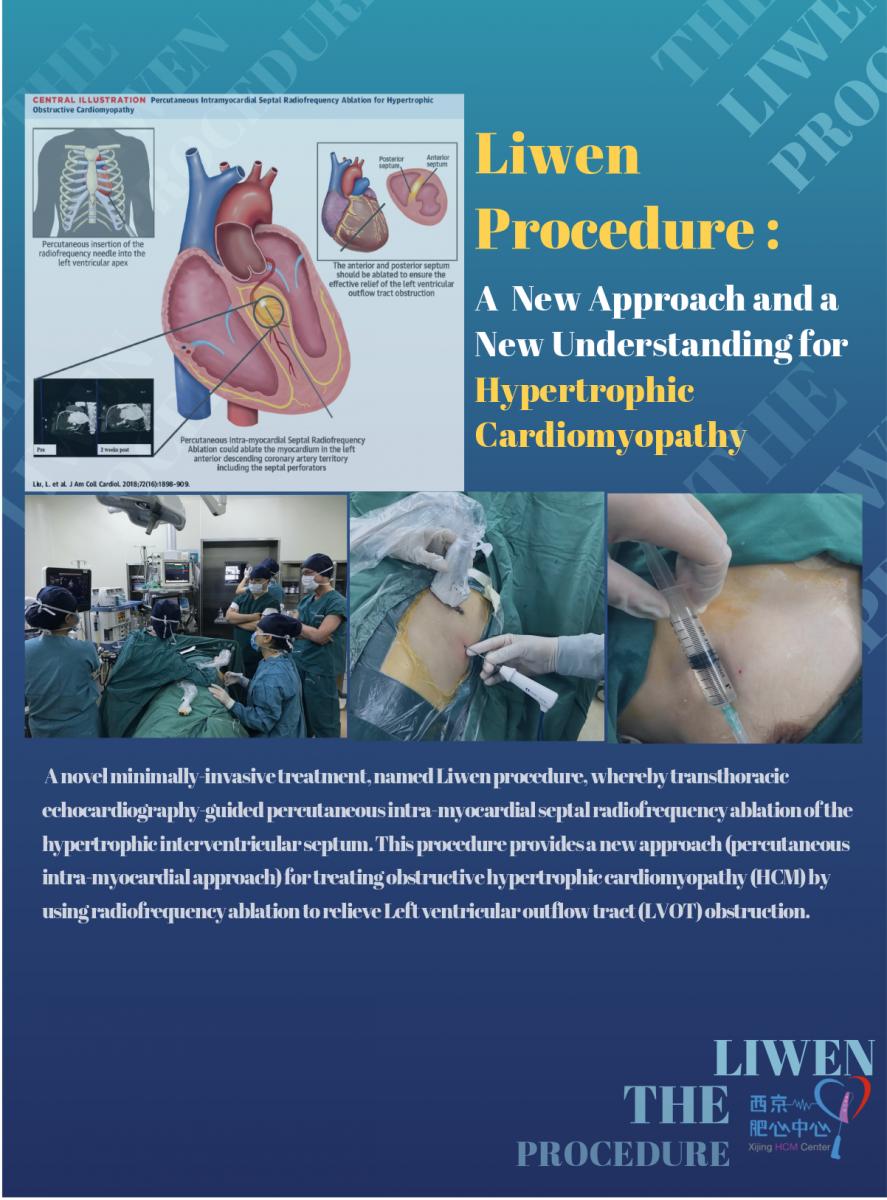
Liwen Procedure: a new approach and a new understanding for hypertrophic cardiomyopathy
VENUS medtech | STRUCTURAL
Impact outline: We developed a novel minimally-invasive treatment, named Liwen procedure, whereby transthoracic echocardiography-guided percutaneous intra-myocardial septal radiofrequency ablation of the hypertrophic interventricular septum. This procedure provides a new approach (percutaneous intra-myocardial approach) for treating obstructive hypertrophic cardiomyopathy (HCM) by using radiofrequency ablation to relieve Left ventricular outflow tract (LVOT) obstruction. By now,our team have finished 103 cases of Liwen Procedure. Most patient recover quickly and quality of life improved.
Background/supporting information: LVOT obstruction has been reported to occur in nearly 70% of patients with HCM and can lead to dyspnea, chest pain, atrial fibrillation, and even sudden cardiac death. Removal of LVOT obstruction has been shown to alleviate symptoms and improve prognosis. Two invasive methods, surgical myectomy and alcohol septal ablation (ASA), are used to relieve LVOT obstruction. Although both procedures can clearly improve clinical symptoms and reduce the LVOT gradient, it is important to consider that sternotomy circulation are required in myectomy, the anatomic variability of the vascularized hypertrophic septum might cause issues in ASA. To take advantage of surgical myectomy and ASA and to avoid sternotomy, reliant on coronary anatomy and damage to the conduction system distributed underneath the endocardium, we proposed a percutaneous intra-myocardial, non-transaortic and non-transcoronary approach. It could reduce LVOT obstruction and avoid sternotomy, reliance on alcohol injection and avoiding damages to the conduction system distributed underneath the endocardium. This was accomplished by inserting a radiofrequency needle and ablating the myocardium of the left anterior descending (LAD) coronary artery distribution including the septal perforators. The localized “therapeutic infarction” was induced by ablation energy delivery in the LAD artery distribution with minimal injury to the surrounding tissues.
* * *
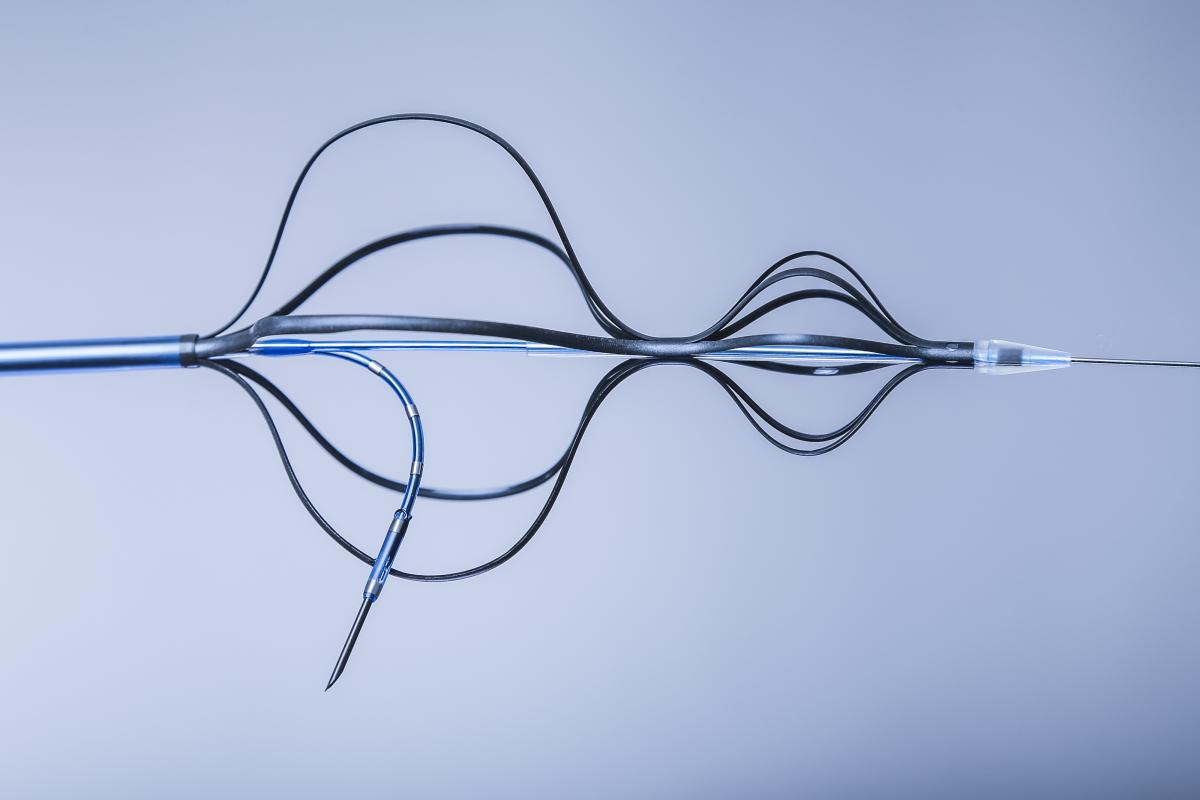
kapsus - NEXT GENERATION TRANSSEPTAL pUNCTURE. SAFE - EASY - ACCURATE.
bavaria medizin | STRUCTURAL
Impact outline: The KAPSUS transseptal puncture system is a sheathed device with a pre-loaded puncture needle that allows a controlled transseptal puncture from the right atrium to provide left atrial access. It is compatible with a 12Fr introducer sheath and a maximum guide wire size of 0.025". Key elements of the device such as tip, housing and needle are visible under fluoroscopy and echo (TEE and ICE).
The device is introduced from the venous side and advanced until its distal tip is positioned in the superior vena cava (SVC). As soon as the catheter's distal tip is accurately placed in the SVC, unsheathing of the device leads to an automatic anchoring in the SVC and in the right atrium. Now, the physician can use the handle in order to adjust the desired angle of attack for the subsequent puncture. Once the correct position and angulation have been found, the needle can be safely and accurately deployed and the puncture can be started
Background/supporting information: Procedures requiring transseptal punctures, such as Mitral Valve Repair/Replacement, LAA Closure and AF Ablation, are becoming more and more common. The KAPSUS will allow an even safer performance of such procedures in the future, as it minimizes the use of imaging techniques and reduces the time required for physician training as well as for the procedure itself.
The transseptal puncture approach involves the catheterization of the right atrium and a subsequent penetration of the septum. This procedure is safer than approaching the left atrium through the left ventricle. Transseptal punctures can be performed for diagnostic and therapeutic purposes with minimal risk if performed accurately. However, potential complications, such as aortic root puncture or tamponade, might occur.
The KAPSUS allows puncturing with fewer steps and, above all, a safe puncture. Once the housing is deployed, the physician can safely release the device without having to fear to lose the position.
The KAPSUS takes advantage of multiple entry points and relies on reproducible methods to locate the puncture site. Its housing ensures consistency of procedures and, at the same time, manages the variability between patients.
* * *

The future of LAAC - WATCHMAN FLX. The feel. The seal. The heal.
Boston scientific | STRUCTURAL
Impact outline: Boston Scientific received the CE Mark for the WATCHMAN FLX LAAC device on February 28th 2019. After a journey of over 55,000 hours of engineering time, dedicated cross team commitment and extensive investment, we are delighted that this new and innovative device is now available to treat nvAF patients across Europe. This is a big milestone for Boston Scientific. The Limited Market Release in 21 centers across Europe is currently ongoing.
* * *

The Occlutech Atrial Flow Regulator (AFR): A Novel Device to Address Unmet Needs
occlutech | STRUCTURAL
Impact outline: Approximately 26 million adults worldwide suffer from heart failure. People with heart failure experience reduced exercise capacity and shortness of breath, even during daily activities. Many advanced stage (NYHA Class III and IV) heart failure patients remain symptomatic despite receiving best available medical therapy. The Occlutech AFR is an investigational device designed to reduce the pressure in the left side of the heart by maintaining a controlled interatrial septal shunt. The device is intended to maintain the shunt that allows blood flow from high pressure left atrium to low pressure right atrium. Early stage clinical trial data are showing that, after implantation of an Occlutech AFR using minimally invasive procedures to create a left to right shunt, decompression of the left heart causes symptomatic relief and improves patients’ quality of life.
Background/supporting information: The Occlutech AFR is in clinical development for two indications:
1. Heart failure (HFrEF and HFpEF): maintain a left-to-right shunt to reduce left atrial pressure (LAP)
2. Pulmonary hypertension (PAH): maintain a right-to-left shunt to reduce the right atrial pressure (RAP)
Initial results of the PRELIEVE Trial have been presented at EuroPCR 2019 and are published in EuroIntervention. The findings demonstrate:
- 100% device success;
- 100% device patency at 3 months;
- Decrease in NYHA class in both HFrEF and HFpEF groups; and
- Improved 6-minute walking test (6MWT) in both HFrEF and HFpEF groups.
Occlutech AFR devices are also being provided to patients with heart failure, pulmonary hypertension, or with failing Fontan indications and who have exhausted all approved treatment options. For these patients, the device is offered under compassionate use grounds with the requisite approvals from local authorities. Since 2015, over 100 such procedures have been performed in 15 countries including the U.S., Canada, and in several European and Asian countries. As of the date of this report 86% of patients are alive and well, 12 patients passed, and two patients are lost to follow-up.
In 2017, Sivakumar et al., published compassionate case data collected from 12 patients with severe PAH and presenting with syncope and right heart failure. All procedures were successful, without any major complications, and all patients had complete relief from syncope. The 6MWT improved significantly from 377 to 423 meters and cardiac index (2.3 to 2.8 l/min/m2) and systemic oxygen transport (367 to 428 ml/min/m2) also showed significant improvements. The device remained patent in all patients at a median follow-up of 189 days (range 10–296 days) resulting in a significant reduction of oxygen saturations from 98% to 85% after exercise.
* * *