
CSI HIGHLIGHT OF THE YEAR award:
imaging
CSI Awards celebrate highlights of the year, events such as first-in-mans, trials, studies and approvals, among others, within the field of congenital, structural and valvar heart disease interventions and interventional imaging.
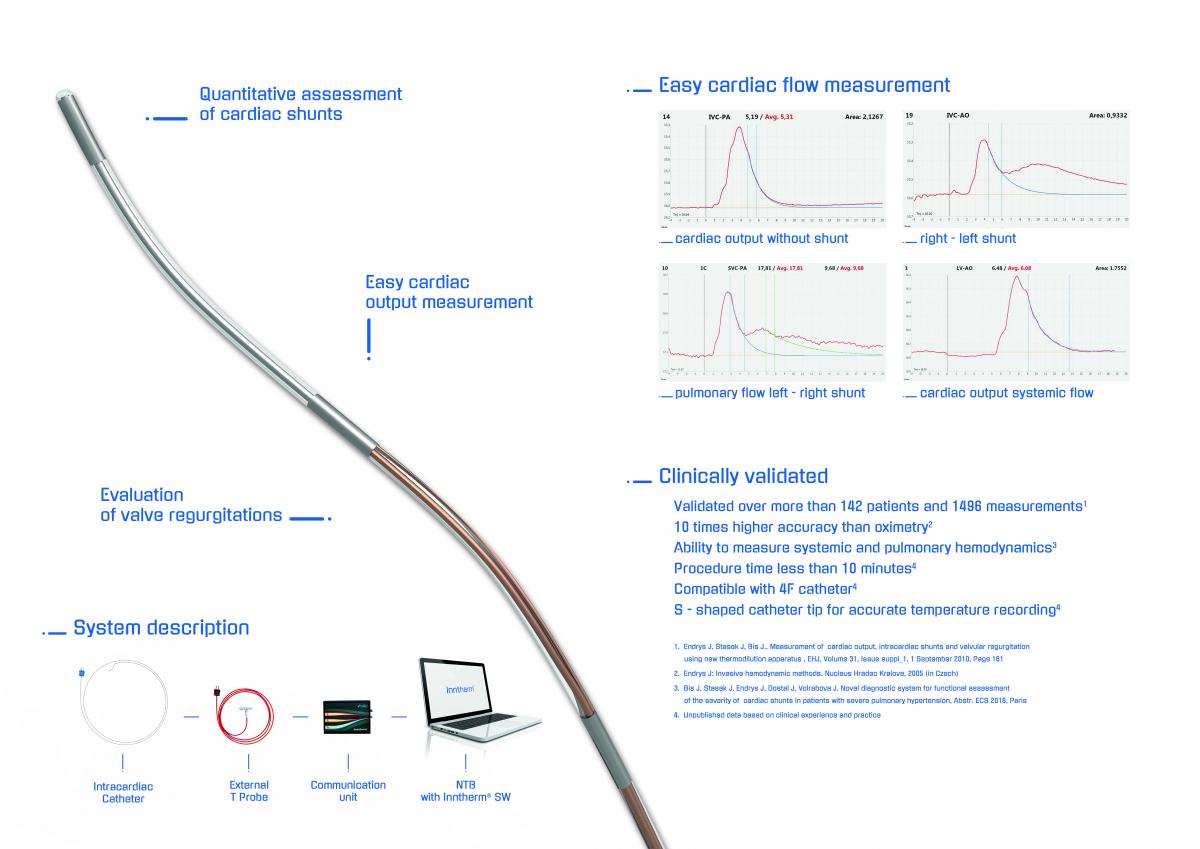
Cardiac Flow Measurement - Functional Assesment of Cardiac Shunts And Other Hemodynamic Parameters
innova medical | imaging
Impact outline: To diagnose severity of cardiac shunts is complicated and inaccurate. Echo and MRI are semiquantitative. Dye dilution is not used any more. Oximetry is inaccurate and complicated to perform. Indication for shunt closure is currently based on indirect figures, anecdotal evidence and personal experience. We developed an innovative system capable of measuring severity of cardiac shunts as well as other hemodynamic parameters. The system consists of three parts – intracardiac catheter, connection box, and computer with dedicated software. The system has been clinically evaluated in hundreds of patients, and results of the FIM study has been published with great success and feedback in major cardiology congresses. It allows to measure many hemodynamic parameters with unequivocal accuracy. It has incomparable clinical advantages to any other technology currently available. The greatest clinical advantage includes cases with severe pulmonary hypertension or LV/RV dysfunction, complex cardiac cases, small children and patients with previous cardiac surgery. Another indication is evaluation of primary pulmonary hypertension and differential diagnoses of the cyanoses. CE mark has been obtained in 2016, and system is reimbursed in Czech Republic and Slovakia. The device was also selected as finalist in ICI Innovation Award in 2017 and TCT Innovation Award in 2018.
Background/supporting information: Scientific background of CFM lies in the combination of two methods. Dye dilution and thermodilution with development of proprietary catheter allowing anatomically access any location in the circulatory system and the heart. The method has been developed by one of the pioneers of interventional cardiology, prof. Jiri Endrys. He used validated principles of dye dilution as diagnostic agent for assessment of cardiac shunts, and adopted the method to cold saline instead of the dye. This led to very simple, easy and cheap procedure. Additionally, especially developed proprietary catheter allowing any anatomical reach within the cardiovascular system led to ability assess any cardiac shunt regardless of anatomical location, or direction of shunt. Development of special software helped to navigate during procedure and improved the accuracy of measurement. The system even allows quantification of shunts in patients with pulmonary hypertension, and measure several other hemodynamic parameters not being able to obtain easily up to date, such as pulmonary vascular resistance. The system was validated vs. standard S-G catheter with correlation coefficient r=0,954, and it is 10 times more accurate than oximetry. Results from several pivotal studies were presented in different congresses, and symposia such as ECS, EUROPace, TCT and CSI.
* * *
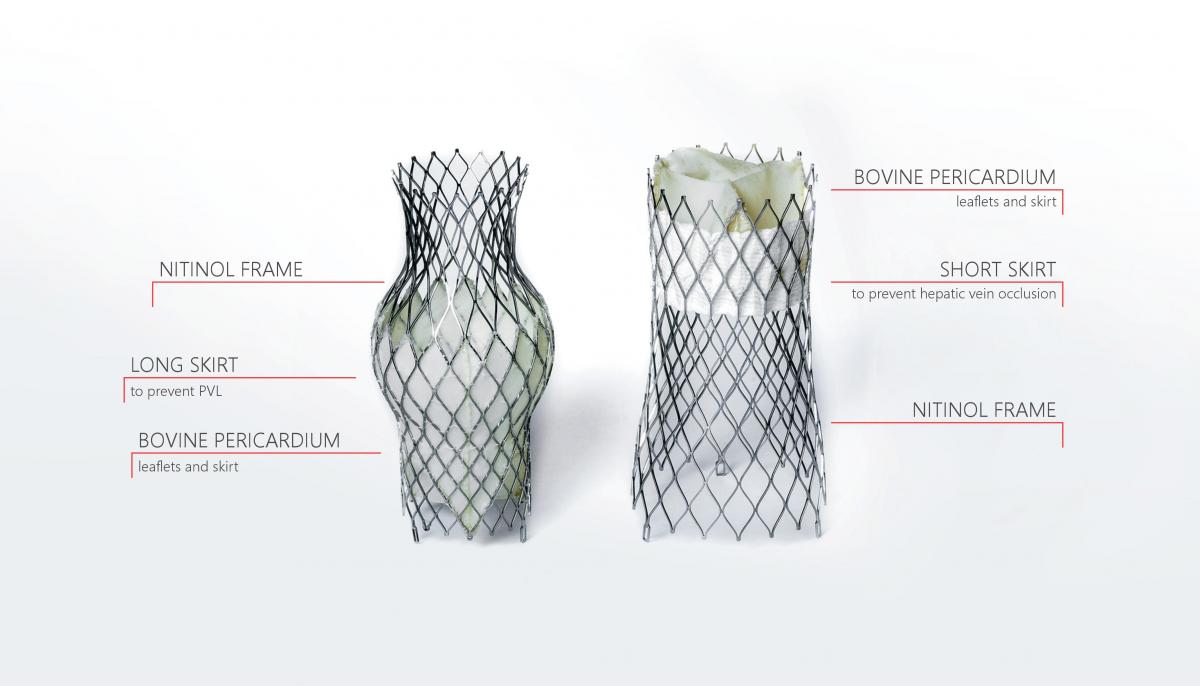
TricValve® Transcatheter Bicaval Valves System - the only complete solution for treatment of tricuspid regurgitation
P+F Products + Features GmbH | IMAGING
Impact outline: TricValve® Transcatheter Bicaval Valves System – the only complete solution for treatment of tricuspid regurgitation using CAVI, specifically designed for the venous system, which is minimally invasive and addresses both SVC and IVC. TricValve® Transcatheter Bicaval Valves is a system of two self-expanding biological valves for the treatment of patients with hemodynamically relevant tricuspid insufficiency and caval reflux. The prostheses are implanted percutaneously into the inferior and superior vena cava without disturbing the native tricuspid valve. It is especially intended for use for patients at extreme risk or who are inoperable for open surgical therapy. It comes exclusively pre-mounted.
Background/supporting information: Tricuspid regurgitation is mainly caused by right ventricular (RV) enlargement with annular dilatation. The implantation of self-expanding valves into the superior (SVC) and inferior (IVC) vena effectively reduce backward regurgitant flow and increases cardiac output. CAVI does not address TR itself but the regurgitation of blood into the caval veins, a condition found often in patients with severe, long-standing TR and RV enlargement. As the RA serves as a reservoir limiting venous backflow hemodynamic proof of caval regurgitation is essential prior to valve implantation. CAVI may result in an increase in RV-afterload by exclusion of backward regurgitation into the caval veins, thus potentially leading to a timely limited impairment of RV-function. Therefore, this approach should be applied in patients with preserved RV-systolic function and normal pulmonary vascular resistance. (Lauten et al. Circ Cardiovasc Interv. 2014;7:268-272).
* * *
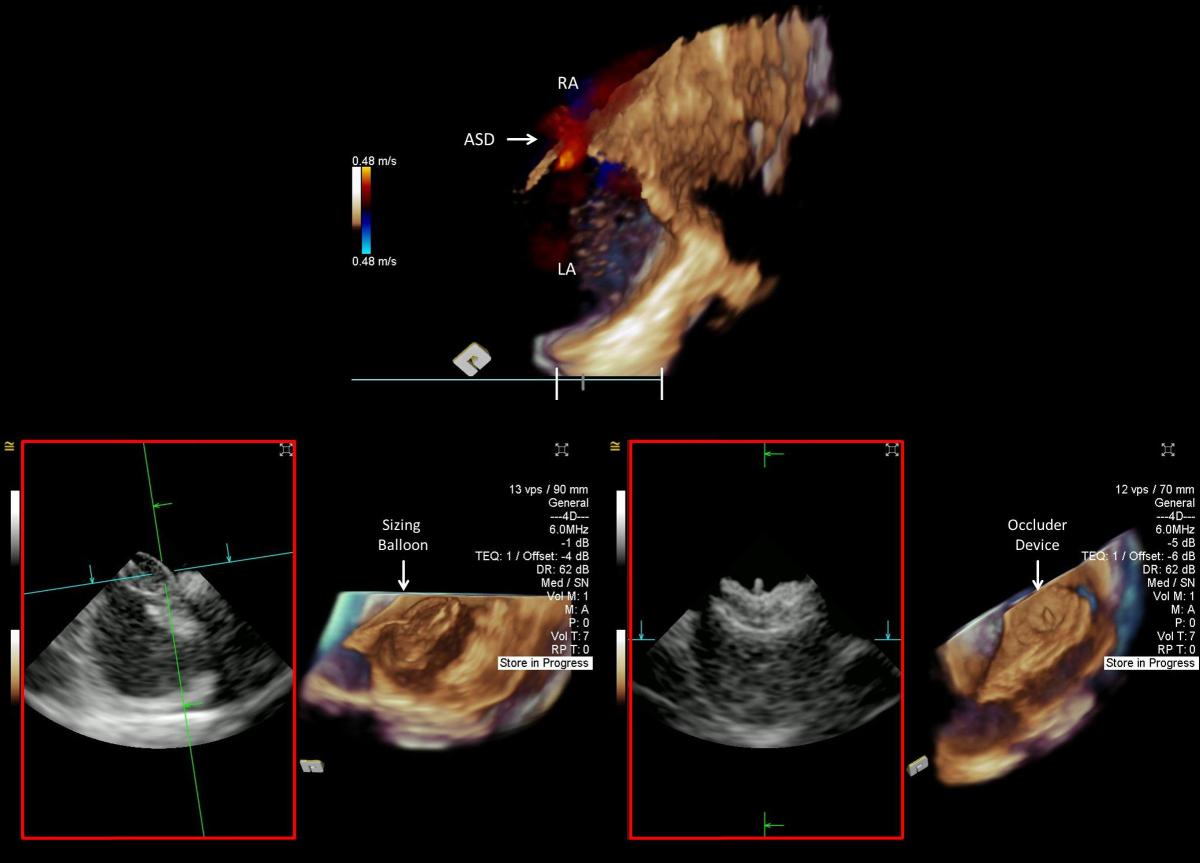
4D Volume ICE Guidance for Transcatheter Device Closure of Atrial Septal Defect
siemens | IMAGING
Impact outline: Echocardiographic imaging is recommended by the American College of Cardiology for guiding transcatheter device closure of inter-atrial septal defect (ASD), including patent foramen ovale (PFO), to prevent paradoxical embolism. Compared to surgical closure, the transcatheter approach is less invasive with faster recovery and up to 45% reduction in recurrent stroke risk. Two-dimensional (2D) intracardiac echocardiographic (ICE) guidance has shown to circumvent limitations associated with the use of transesophageal echocardiographic guidance such as the need for general anesthesia. 2D-ICE only offers a single plane of imaging. With four-dimensional (4D) volume ICE, multiplanar imaging of the anatomical landmarks improves spatial orientation and ASD assessment to determine appropriate device selection. The new generation of 4D volume ICE offers a larger field-of-view for optimal visualization of the target objects and guidance of the sizing balloon and the occluder device. Volume color Doppler can be utilized to evaluate the interatrial flow pre- and post-closure. Visualization of capture of the interatrial septal rims within the device is enhanced by volume ICE. With improved navigational confidence, fluoroscopic imaging can be limited to brief intermittent assessment reducing radiation exposure. Volume ICE imaging can be performed by the interventional cardiologist with less reliance on the echocardiologist or additional staff.
Background/supporting information: Stroke is a leading cause of mortality and morbidity with 87% ischemic in nature. About one-third of ischemic strokes have unclear etiology; PFO is present in 40% of these patients and may cause stroke via a paradoxical embolism. Transcatheter device closure of PFO has been shown to reduce the risk of recurrent ischemic stroke with moderate benefits compared to antiplatelet therapy, and faster patient recovery compared to surgery. Transcatheter interventional procedures have increasingly relied upon echocardiographic imaging, with the use of 4D technology and its multiplanar volume acquisition, for precise intraprocedural imaging guidance. While transesophageal echocardiography requires additional support and general anesthesia, intracardiac echocardiography can be performed by a single operator under conscious sedation. Proper patient selection and optimal imaging guidance are paramount to successful closure of PFO/ASD.
* * *
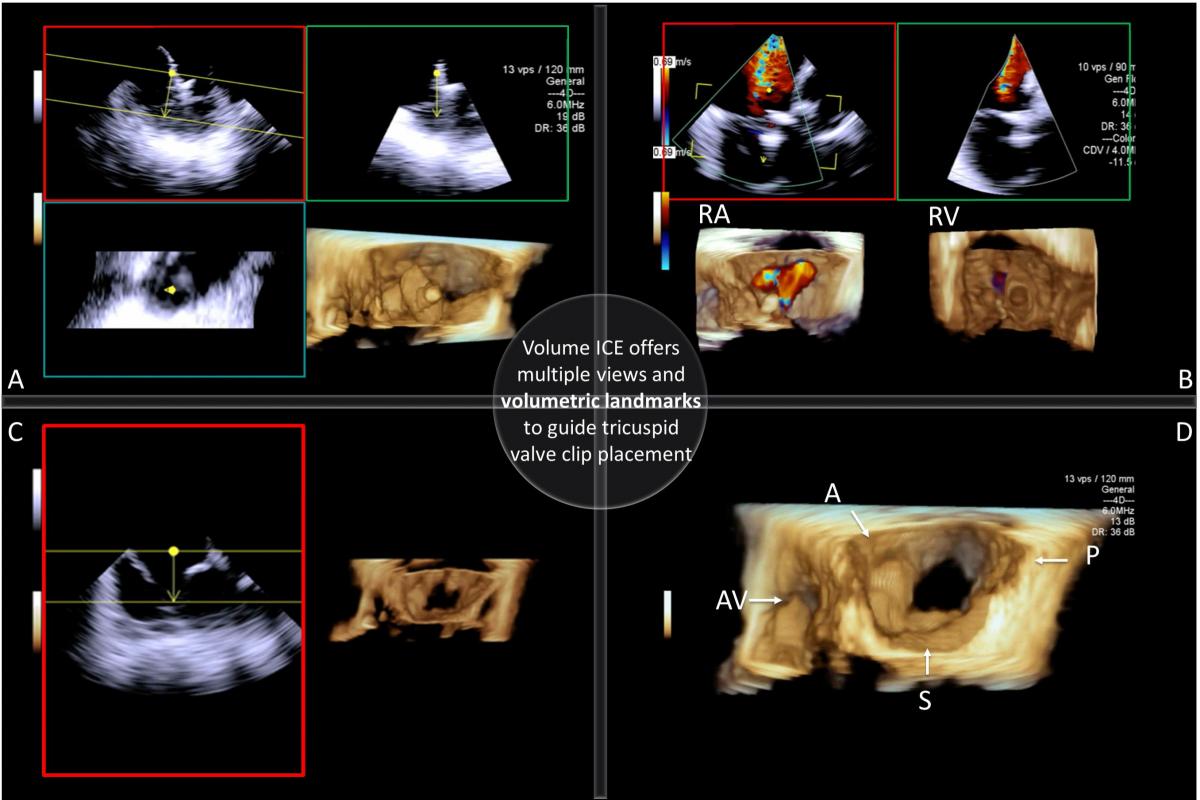
4D Volume ICE Guidance for Transcatheter Tricuspid Valve Leaflets Intervention
SIEMENS | IMAGING
Impact outline: Tricuspid regurgitation (TR) is associated with adverse prognostic implications and high surgical mortality for isolated tricuspid valve (TV) intervention. Emerging transcatheter TV interventions with early feasibility studies have shown variable reduction in TR. We report our early clinical experience with transcatheter tricuspid valve leaflet intervention (TTVLI) using large field-of-view volume intracardiac echocardiography (ICE) for intraprocedural imaging guidance. With volume ICE from the right atrium, the TV leaflets can be viewed on multiple imaging planes (Figure A) and en face from the right atrium (RA) and the right ventricle (RV) simultaneously with color Doppler overlay to quantify TR (Figure B). The TV segment of interest can easily be cropped, refined and displayed in the volume (Figure C). By identifying the anatomical landmarks on the volume (Figure D) and methodically aligning the imaging planes with the target leaflets and the clip, optimal views can be achieved to guide adequate clip implantation. Advanced imaging techniques are key to procedural success and real-time volume ICE offers a novel workflow for TTVLI.
Background/supporting information: The structural complexity and imaging difficulty of the right heart has limited our understanding of the right heart failure compared to its left counterpart. Studies suggest that patients undergoing transcatheter TV interventions are in the late stage of the disease with high-risk clinical profile and torrential TR. Proper patient selection and understanding of TV anatomy are essential to maximize benefit from TR correction. The American College of Cardiology recommends transcatheter TV interventions for symptomatic patients with moderate to severe (3+) and severe (4+) tricuspid regurgitation who are deemed to be at high risk for conventional surgery. In this study, four patients with 3-4+ TR and heart failure symptoms were selected for transcatheter TV leaflet intervention. Echocardiographic imaging guidance was performed with TEE and volume ICE. The ICE catheter was placed in the right atrium for TTVLI intraprocedural imaging guidance. The average procedure time was 2 hours and a 2 grade TR reduction was achieved post TTVLI. At 30-days follow-up, all of the 4 patients showed remarkable symptom improvement including marked reduction in peripheral edema.
* * *
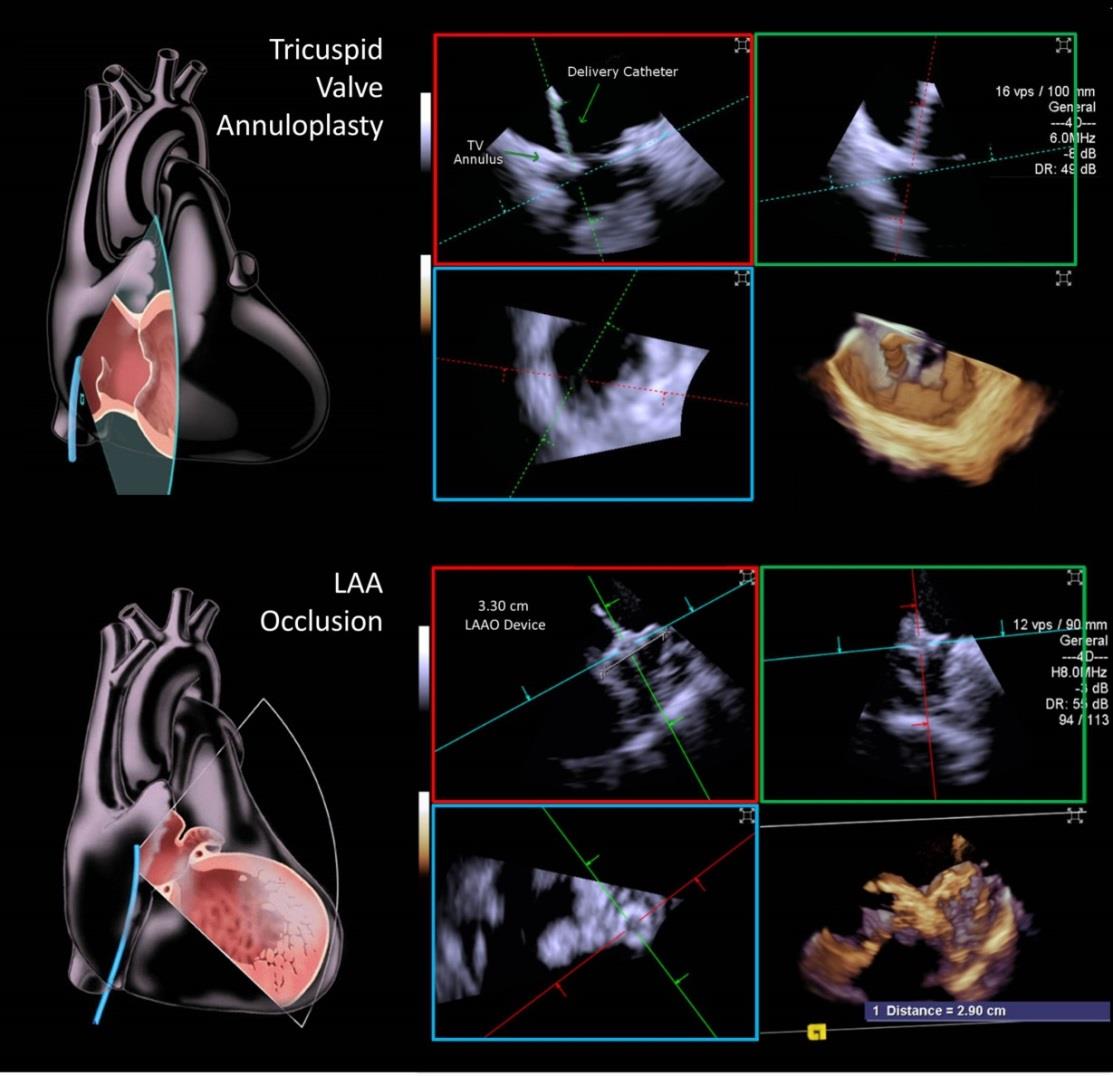
4D Volume ICE Guidance for Transcatheter Structural Heart Interventions
SIEMENS | IMAGING
Impact outline: Transesophageal echocardiography (TEE) remains the gold standard for most structural heart interventions but requires anesthesia and endotracheal intubation. Two-dimensional intracardiac echocardiography (ICE) has been limited for structural interventions but recent advancement in ICE, with large field-of-view and four-dimensional (4D) imaging capability, has expanded the current paradigm. We report our early clinical experience with 4D volume ICE guidance for transcatheter tricuspid valve (TV) annuloplasty and left atrial appendage occlusion (LAAO). TEE has limited ability to visualize TV due to its off-line position. Transcatheter TV annuloplasty can be successfully performed using 4D volume ICE from the right atrium for visualization of TV leaflets and annulus with diagnostically adequate spatial and temporal resolution. Optimal imaging of the left atrium and LAA requires the ICE catheter to be placed in the LA transseptally. Transcatheter LAAO can be successfully performed under conscious sedation using 4D ICE only for intraprocedural measurements and guidance. There is a need to better image the heart from the interior; TEE’s requirement to anesthetize and intubate the patient adds to procedural morbidity, expense, and complexity. Volume ICE has the potential to eliminate some of these aspects of imaging and make the over-all procedural experience less complex and less invasive for patients.
* * *
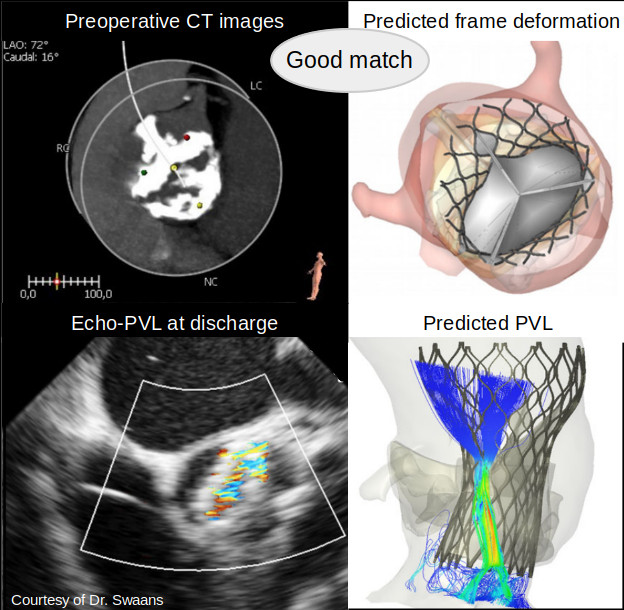
Can patient-specific computational simulation predict PVL and conduction disturbance after TAVR in bicuspid aortic valve patients?
feops | IMAGING
Impact outline: We propose patient-specific computational simulations to virtually deploy a transcatheter aortic valve in patient-specific anatomy, to support pre-operative planning with indications of possible complications, as PVL and conduction abnormalities. The simulations allow to evaluate the interaction between the device and the anatomy, with a particular focus on patients with bicuspid aortic valve. The computational simulation tool can be ideally used for all patients, but it has been optimized and improved to be able to simulate the anatomical complexity of BAV patients, who can mostly benefit from an optimal preoperative planning, due to the high anatomical variability and potentially younger age. The patient-specific computational modelling adequately predicts feasibility and outcome of TAVR in patients with BAV disease. Moreover, it improves decision-making and, potentially, individual procedural outcomes in this difficult patient population.
Background/supporting information: The recently CE-mark-cleared FEops HEARTguide platform allows clinicians to submit the CT images of the patients scheduled for TAVR procedure for a virtual deployment of the selected device. The patient-specific computational simulation of TAVR in BAV morphology have been retrospectively validated on a cohort of 37 patients, where the computational simulations were compared to post-procedural computed tomography imaging, cineangiography, echocardiography and electrocardiograms. The computational simulations accurately predicted the transcatheter heart valve frame deformation, more than mild paravalvular regurgitation and significant conduction abnormalities. The use of patient-specific computational simulations can be used to identify optimal heart valve sizing and positioning which can potentially reduce paravalvular regurgitation and/or conduction disturbance.
* * *
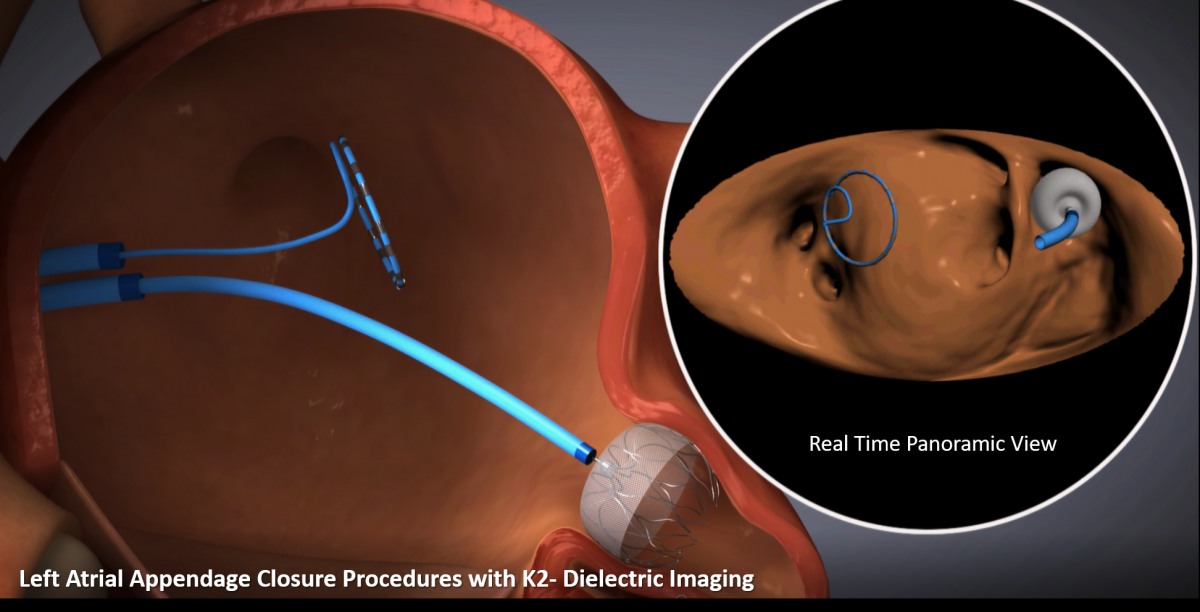
K2-Dielectric Imaging – A Catheter based Hybrid Imaging modality for Transcatheter Therapies for Structural Heart Disease
hobart | IMAGING
Impact outline: K2 Dielectric Imaging (DI) enables structural heart interventionalists to image device implantation procedures from access to validation. K2 DI presents real time, HD imaging for structural heart intervention, using conventional disposable 3F multi electrode catheters. The imaging catheter acquires a 4 dimensional, hybrid anatomical-functional image of the heart. K2 DI has the potential to reduce need for TEE, fluoroscopy and anesthesia. In addition, the interventionalist controls the imaging technique (vascular access, trans-septal and implant navigation and validation). K2 DI is designed to shorten procedures and potentially reduces ionizing radiation, remove the need for contrast agent and empower the interventionalist to take control of the procedure pathway.
Background/supporting information: Transcatheter therapies for both congenital and noncongenital structural heart lesions have continued to expand and evolve rapidly in the past three decades. The evolution of percutaneous treatments requires the understanding of 3D anatomy as well as the need for soft-tissue visualization. Imaging has become an indispensable part of the SHD procedure but with high complexity and limitations. A variety of imaging modalities are used, often in tandem, and include fluoroscopy, angiography, 2D and 3D transthoracic, intracardiac, transesophageal echocardiography, cardiac CT and CMR.
Recently, DI imaging was launched for cardiac electrophysiology (KODEX-EPD system imaging, Philips Medical Systems). K2 DI is an Imaging modality specifically designed for each transcatheter therapy. K2 DI uses reconstructed images based transmission and reflection of wide band radio waves transmitted and received from the imaging catheter.
* * *